Key Takeaways
- A new medication to treat advanced cancer was developed by Dr. Matthew Taylor, physician-scientist at Providence Cancer Institute.
- A phase I clinical trial will evaluate the effectiveness and safety of the medication, called M3T01. This is a leading-edge study.
- The clinical trial is currently enrolling people with advanced cancers at Providence Cancer Institute.
- M3T01 blocks Fas ligand, making the immune response stronger and longer lasting. This approach could boost the power of immunotherapy.
-
The development of M3T01 was made possible because of generous donors.

When Matthew Taylor, M.D., joined Providence Cancer Institute in 2019, he brought a bold idea: What if there was a medication to help protect T cells from being destroyed by cancer?
Dr. Taylor believed there was a way to help T cells—our immune system’s frontline fighters—survive longer inside tumors, where they’re often shut down by a death signal called Fas ligand. His approach was to develop a medication that blocks that signal, shielding T cells from destruction and allowing them to keep attacking cancer.
Backed by Walter Urba, M.D., Ph.D., Dr. Taylor’s concept gained traction. Dr. Urba is a pioneer in immunotherapy and former chief medical officer at Providence Cancer Institute and director of cancer research at Earle A. Chiles Research Institute, the research arm of Providence Cancer Institute.
Now, with the support of generous donors, Dr. Taylor’s idea has emerged as a new medication: M3T01. It’s currently being evaluated in a phase I clinical trial for people with advanced cancers. The study is actively enrolling patients at Providence Cancer Institute.
What is so special about Fas ligand?
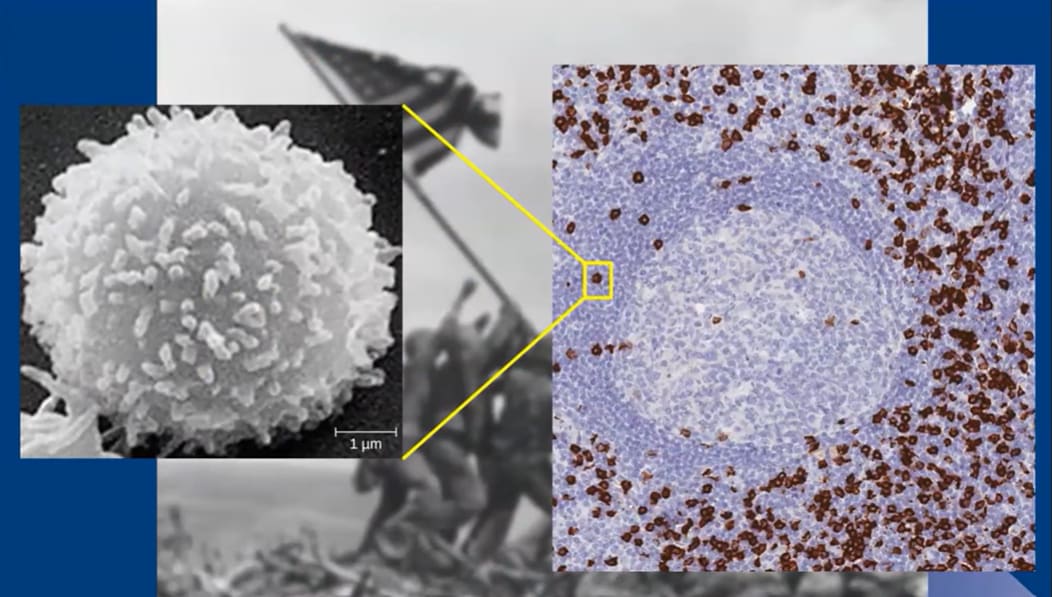
Fas ligand, also known as FasL, normally plays an essential role in keeping the immune system balanced. When the body detects a viral or bacterial threat, it rallies an army of T cells to respond. After the infection is neutralized, FasL acts like a cleanup crew, signaling for the excess T cells to be cleared away. This process helps rebalance the immune system, keeping it ready for future attacks.
But cancer can hijack this fine-tuned system. Tumors can use FasL to destroy the T cells sent to fight them. Furthermore, cancer can draft this same protein, which is normally part of a healthy immune response, to promote tumor growth and spread.
Understanding the complex activities of FasL helped shape Dr. Taylor’s approach to developing a new type of therapy. “Over the past couple of decades, we’ve found several ways to activate T cells [using immunotherapy],” he says. “The challenge is keeping them alive once they reach the tumor,” he says.
While existing immunotherapies like PD-1 and CTLA-4 inhibitors (e.g., pembrolizumab or ipilimumab) work by reactivating “exhausted” T cells, they don’t stop those cells from being killed off.
Blocking FasL supports the immune response, making it not only stronger but also longer lasting. This strategy could boost the power of immunotherapy in treating advanced cancers.
Developing a new therapy at Providence
Within a year after joining Providence, Dr. Taylor’s vision for a new cancer therapy was underway in the lab at Earle A. Chiles Research Institute. But creating a new and safe medication is a long and complex process.

The first step was to develop the medication. Dr. Taylor and his research team spent three years exploring monoclonal antibodies, engineered proteins designed to block the FasL protein. By masking FasL, the antibody helps prevent T cells from being destroyed so they stay focused on attacking the tumor.
Once the antibody for M3T01 was developed, it was manufactured and then tested for safety. Finally, M3T01 was submitted to the Food and Drug Administration (FDA) as an Investigational New Drug (IND), an essential approval step before launching a clinical trial.
In January 2025, the FDA approved M3T01 for clinical testing.
So far, only one FasL-blocking medication, called asunercept (APG101/CAN008), has advanced to cancer clinical trials. Although it showed some promise in treating people with glioblastoma and myelodysplastic syndrome, its impact was limited.
M3T01 was designed to bind more effectively to FasL and offer better protection to T cells. In preclinical studies, M3T01 was more than 300 times as potent as APG101.
Phase I trial underway
With FDA clearance secured, the first clinical trial of M3T01 is underway at Providence Cancer Institute. The study will evaluate the medication in people with cancer that has metastasized or cannot be removed by surgery.
There are two parts to the study: Part 1 is to evaluate the safety and maximum tolerated dose of M3T01 by itself and in combination with pembrolizumab, another immunotherapy. Part 2 is to assess the safety of M3T01 given in combination with pembrolizumab and chemoradiation or pembrolizumab and FOLFOX.
The outcome of part 1 will determine the highest dose that can be given safely and the recommended dose in the next study phases.
“We’re combining M3T01 with pembrolizumab because we need to both activate the T cells so they can effectively fight cancer and protect them to keep them alive,” says Dr. Taylor. “That's why we think M3T01 in combination with immune checkpoint inhibitors is where we will find the greatest efficacy.”
Once the optimal dose of M3T01 is established by itself, the study moves to Part 2, where M3T01 will be tested for the following cancers:
- Newly diagnosed glioblastoma. (Patients will be treated with M3T01 in combination with temozolomide chemotherapy and radiation therapy.)
- Newly diagnosed gastric or esophageal adenocarcinoma. (Patients will be treated with M3T01 in combination with FOLFOX chemotherapy plus pembrolizumab.)
- Recurrent or metastatic head and neck squamous cell carcinoma. (Patients will be treated with M3T01 plus pembrolizumab.) This stage will determine how well the combination of treatments works across specific cancer types that remain difficult to treat.
The information gained from this phase I study will help Dr. Taylor and his research team determine protocols for a phase II study, which is a larger study designed to test effectiveness of M3T01.
A legacy of leading the way in research
For patients facing hard-to-treat cancers, access to promising new therapies can make all the difference. At Providence Cancer Institute, the development of M3T01 reflects a long-standing commitment to expanding those options and pushing the boundaries.
“For over 30 years, Providence Cancer Institute has been a pioneer in developing novel cancer therapies including immune checkpoint inhibitors, cancer vaccines, and adoptive cell therapies,” says Dr. Taylor.
This type of work happens in a uniquely resource-rich setting where patients benefit directly from forward-thinking science and access to novel therapies as soon as they’re ready for clinical testing.
“The FasL project is exactly the kind of thing that this institute was founded to do; translate new discoveries from the laboratory to the clinic where they can benefit patients. The fact that this is happening in Oregon, not Boston, Houston or Seattle, is a testament to our team and continued commitment to provide Oregonians world-class care and promising clinical trials close to home,” says Bryan Bell, M.D., D.D.S., executive medical director of Providence Cancer Institute and director of the Earle A. Chiles Research Institute.
New treatments are made possible primarily through philanthropy, which helped launch the early development of M3T01. As Dr. Taylor puts it, without donor support, the early work that led to this phase I trial wouldn’t have been possible. It’s a reminder that scientific momentum and breakthroughs in patient care depend on a powerful combination of vision, determination and community investment.
Do you want to learn more about the study or how to refer a patient? Visit the Providence Research Network to see this study and other clinical trials.
About Providence Cancer Institute of Oregon
 Providence Cancer Institute is a leading provider of cancer care in Oregon and a global leader in immuno-oncology. Through its research division, the Earle A. Chiles Research Institute, patients have access to state-of-the-art genomic sequencing, adoptive cellular therapies and a robust clinical research portfolio comprising early phase, investigator-initiated, cooperative group and industry-sponsored trials. Learn more at providence.org/ORcancer.
Providence Cancer Institute is a leading provider of cancer care in Oregon and a global leader in immuno-oncology. Through its research division, the Earle A. Chiles Research Institute, patients have access to state-of-the-art genomic sequencing, adoptive cellular therapies and a robust clinical research portfolio comprising early phase, investigator-initiated, cooperative group and industry-sponsored trials. Learn more at providence.org/ORcancer.
Subscribe to Finish Cancer Through Research newsletter to follow science breakthroughs reshaping cancer care at Providence Cancer Institute.
Related news
A new generation CDK4 inhibitor study for people with HR+ HER2- breast cancer
Providence researchers share cancer discoveries at 2025 ASCO meeting





















